- Home
- Latest News
- Squaring the Circle
Squaring the Circle
Kalpesh Shah of Veolia Water Technologies (Veolia) takes a 360° look at water in the pharmaceuticals industry
A study of the UK pharmaceutical industry by the University of Hertfordshire in 2013 found that “because water use is viewed as a comparatively inexpensive resource, this undermines investment in water efficient technologies”. This is no surprise. Mention “water” in the same sentence as “pharmaceutical production” and most pharmaceutical engineers immediately think of compendial Purified Water and Water for Injection (WFI). Since both are critical and, relatively, expensive ingredients in the industry’s products, this is quite reasonable but they shouldn’t be considered in isolation.
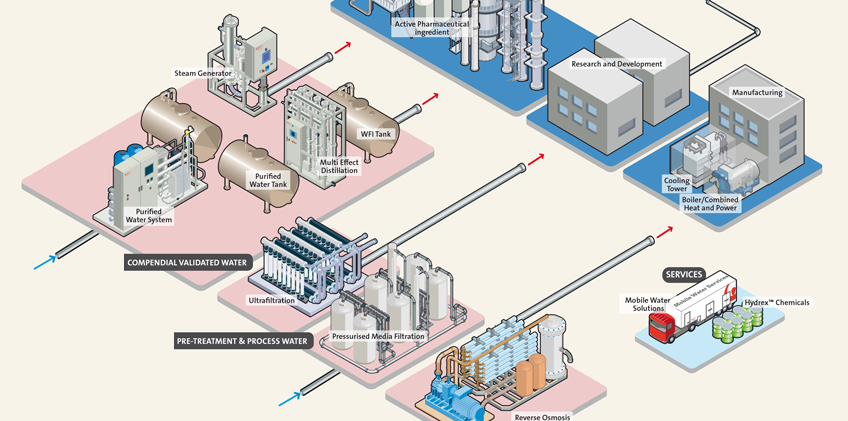
A typical pharmaceutical facility uses water for a variety of purposes and an overall water management approach can be beneficial both environmentally and financially. Most pharmaceutical facilities take their “raw” water from a mains drinking water supply at a cost of approximately £1.20/m3 and then purify it by reverse osmosis (RO) and electrodeionisation, to produce Purified Water at an extra cost of about £1.00/m3. Typical of the “packaged plants” available for producing Purified Water using this technology is the Continuous Deionisation (CEDI) IonPRO LX. Orbis Consumer Products is a contract pharmaceuticals manufacturer operating two production sites in Park Royal, London where they manufacture analgesic and anti-inflammatory suspensions, including products for children. These liquid products are produced using Purified Water which meets the requirements of the European Pharmacopoeia (Ph Eur) by an IonPRO system consisting of a mains water tank, activated carbon filter, duplex softener, primary deionisation by RO and polishing deionisation by CEDI. The resulting treated water is delivered to a storage tank from which it is re-circulated via a UV disinfection unit and ring main supplying the various points of use. For larger systems a range of compendial packaged plants, such as Veolia’s Orion, uses the same basic process of softening, RO and final CEDI with the addition of a tank with a heater for automatic hot water sanitisation of plant and ring main to meet the water quality standards for USP and PhEur Purified Water and PhEur Highly Purified Water. The system is fully compliant with FDA, cGMP and GAMP requirements. Lofthouse of Fleetwood uses this compendial plant for its main Fisherman’s Friend production line, where Purified Water is an ingredient, whilst its new sugar free lozenge line and a new Pharma Suite have an IonPRO LX unit each to supply water for cleaning.
WFI demands distillation of Purified Water, adding a further £1.00p/m3 bringing the total to over £3.00. Egyvac, an Egyptian vaccine manufacturer recently upgraded their water system using an Orion to produce Purified Water and Polaris distillation to upgrade the Purified Water to WFI. The Polaris range includes a clean steam generator and both multiple effect and vapour compression stills, all designed in accordance with GAMP, cGMP, ISPE and FDA guidelines.
Although Purified Water and WFI are essential for manufacturing pharmaceutical products, there are many other equally essential uses of water in a pharmaceutical facility, as (Fig 1) shows. Cleaning in place is an obvious one but let’s not forget the humble factory boiler. Without it, there would be no steam for heating, autoclaves and for running the WFI distillation plant or the clean steam generator. The boiler needs a supply of make-up water, which has to be treated, as does the equally important factory cooling tower. Then there’s water for “domestic” purposes – washrooms, showers and catering. And don’t forget the laboratory that needs ultrapure water for analysis and media preparation.
Using water inevitably means producing wastewater. Aside from the Purified Water and WFI that is discharged to drain, there are the waste streams from the water treatment systems themselves – spent regenerant from softeners and deionisers, reject water from RO plants and blowdown from the still. In addition there is blowdown from the boiler and cooling tower, waste from laboratory sinks and domestic wastewater. Most facilities dispose of this to sewer, in accordance with a consent to discharge and are charged for it in accordance with the Mogden formula. Sewer discharge averages about £2.00/m3 so buying mains water, treating it to WFI standard and discharging it to sewer can cost well over £5.00/m3. But if the wastewater contains Active Pharmaceutical Ingredients (APIs) it may have to be collected and taken off site for incineration or some other disposal route.
A typical 1,000L/h Purified Water RO plant will reject about 30L/h, but a concentrate recovery unit can reduce this significantly. A biotechnology plant located at Ringaskiddy, Co. Cork, a phase 2B/3 clinical trial scale facility: manufactures, purifies, formulates and bulk fills mammalian cell culture derived proteins. The company already had an Orion on site to produce Purified Water and Veolia’s Reco Solutions team was able to use the RecoBLUE online calculator to show the engineers how adding a recovery RO could take advantage of the plant’s ‘waste’ water and turn it into a valuable resource. The plant is now recovering 75% of the Orion’s wastewater (up to 2,700m3 of reclaimed water per year) and this water is recycled back to the plant inlet to begin the purification cycle once again, replacing mains water. The resulting reduction in both mains water and sewer discharge meant that the recover RO paid back in only seven months.
On site biological wastewater treatment using the latest generation of aerobic membrane bioreactors (MBRs), such as a Biosep, with ultrafiltration membranes, has a proven track record in removing some of the more recalcitrant chemicals species that are often present in pharmaceutical wastewater. The resulting reduction in Mogden formula charges can often give a payback in a couple of years. If the wastewater is particularly high in COD it may be economic to invest in a Memthane anaerobic MBR to generate biogas fuel to reduce boiler energy costs. An MBR produces treated water which is very low in suspended solids and bacteria and can often be discharged to a surface water at a significantly lower cost than the sewer. But with this quality, it is clearly better to re-use the water for non-critical purposes such as flushing toilets or to feed it to a RO plant to produce boiler or cooling tower make-up water. Where biological treatment is not appropriate, evaporation can recover around 90% of the water leaving a small volume of concentrated waste for disposal.
If wastewater contains APIs that are either non-biodegradable or toxic to biological treatment plant bacteria, there are options such as Actiflo Carb – combining adsorption and clarification process – or wet air oxidation which produces free hydroxyl radicals to break down even the most complex organic molecules into carbon dioxide and water. Although very expensive, wet air oxidation is becoming increasingly attractive, as alternative disposal routes become more expensive. But evaporation can provide an environmentally friendly disposal route together with an opportunity for recovering these valuable ingredients. The latest generation of heat pump evaporators operate under vacuum, which means that water boils at about 35°C so that even heat sensitive APIs can be recovered. And the option of scraped film technology can handle high viscosity or foaming wastes. A manufacturer of nicotine products installed an Evaled PC R500 scraped film evaporator to process 550 litres per day of waste alkaloid solution to produce 500 litres per day of aqueous condensate, which can be discharged or recycled, and a concentrated stream containing the alkaloid that is then purified for recycling as a pharmaceutical grade API.
So taking an holistic 360° look at the manufacturing facility’s water requirement from compendial water to final waste disposal can lead to better management. Combining the techniques discussed above to re-use and recycle water can reduce a pharmaceutical facility’s water footprint by up to 50% together with substantial cost savings. Good engineering. Good economics. Good sustainability.
Got a question to ask us?